PCA: Difference between revisions
| (38 intermediate revisions by the same user not shown) | |||
| Line 10: | Line 10: | ||
* [https://towardsdatascience.com/how-exactly-does-pca-work-5c342c3077fe How exactly does PCA work?] What PCA (with SVD) does is, it finds the best fit line for these data points which minimizes the distance between the data points and their projections on the best fit line. | * [https://towardsdatascience.com/how-exactly-does-pca-work-5c342c3077fe How exactly does PCA work?] What PCA (with SVD) does is, it finds the best fit line for these data points which minimizes the distance between the data points and their projections on the best fit line. | ||
* [https://www.sartorius.com/en/knowledge/science-snippets/what-is-principal-component-analysis-pca-and-how-it-is-used-507186 What Is Principal Component Analysis (PCA) and How It Is Used?] PCA creates a visualization of data that minimizes residual variance in the least squares sense and maximizes the variance of the projection coordinates. | * [https://www.sartorius.com/en/knowledge/science-snippets/what-is-principal-component-analysis-pca-and-how-it-is-used-507186 What Is Principal Component Analysis (PCA) and How It Is Used?] PCA creates a visualization of data that minimizes residual variance in the least squares sense and maximizes the variance of the projection coordinates. | ||
* [https://www.statforbiology.com/2021/stat_multivar_pca/ Principal Component Analysis: a brief intro for biologists] | |||
= Linear Algebra = | |||
[https://shainarace.github.io/LinearAlgebra/index.html Linear Algebra for Data Science with examples in R] | |||
= R source code = | = R source code = | ||
| Line 67: | Line 71: | ||
# yellow | # yellow | ||
# gray | # gray | ||
== palmerpenguins data == | |||
[https://allisonhorst.github.io/palmerpenguins/articles/pca.html#principal-component-analysis-pca PCA with penguins and recipes] | |||
== Theory with an example == | == Theory with an example == | ||
[http://www.sthda.com/english/articles/31-principal-component-methods-in-r-practical-guide/118-principal-component-analysis-in-r-prcomp-vs-princomp/ Principal Component Analysis in R: prcomp vs princomp] | [http://www.sthda.com/english/articles/31-principal-component-methods-in-r-practical-guide/118-principal-component-analysis-in-r-prcomp-vs-princomp/ Principal Component Analysis in R: prcomp vs princomp] | ||
= PCA and | == Biplot == | ||
* [https://www.statforbiology.com/2021/stat_multivar_svd_biplots/ Biplots are everywhere: where do they come from?] | |||
* ggbiplot. [https://www.datacamp.com/tutorial/pca-analysis-r Principal Component Analysis in R Tutorial] from datacamp. | |||
* [https://www.r-bloggers.com/2023/09/exploring-multivariate-data-with-principal-component-analysis-pca-biplot-in-r/ Exploring Multivariate Data with Principal Component Analysis (PCA) Biplot in R] | |||
== factoextra == | |||
* https://cran.r-project.org/web/packages/factoextra/index.html | |||
* [http://sthda.com/english/wiki/factoextra-r-package-easy-multivariate-data-analyses-and-elegant-visualization Factoextra R Package: Easy Multivariate Data Analyses and Elegant Visualization] | |||
= Properties = | |||
<ul> | |||
<li>Definition. If x is a random vector with mean <math>\mu</math> and covariance matrix <math>\Sigma</math>, the the principal component transformation is | |||
: <math> | |||
y = \Gamma ' (x - \mu), | |||
</math> | |||
where <math>\Gamma</math> is orthogonal, <math>\Gamma' \Sigma \Gamma = \Lambda</math> is diagonal and <math>\lambda_1 \geq \lambda_2 \geq \cdots \geq \lambda_p \geq 0</math>. The strict positivity of the eigenvalues <math>\lambda_i</math> is guaranteed if <math>\Sigma</math> is positive definite. The ith principal component of x may be defined as the ith element of the vector y, namely, as <math> y_i = \gamma_{(i)}' (x-\mu)</math>. Here <math>\gamma_{(i)}</math> is the ith column of <math>\Gamma</math>, and may be called the ith vector of principal component '''loadings'''. | |||
</li> | |||
<li>Properties | |||
* <math> E(y_i) =0, </math> | |||
* <math> Var(y_i) =\lambda_i, </math> | |||
* <math> Cov(y_i, y_j) =0, i \neq j, </math> | |||
* <math> Var(y_1) \geq Var(y_2) \geq \cdots Var(y_p) \geq 0, </math> | |||
* <math> \sum Var(y_i) = tr \Sigma, </math> '''total variation''' | |||
* <math> \prod Var(y_i) = |\Sigma| </math>. | |||
</li> | |||
<li>The sum of the first k eigenvalues divided by the sum of all the eigenvalues, <math>(\lambda_1 + \cdots + \lambda_k)/(\lambda_1 + \cdots + \lambda_p)</math>, represents the '''proportion of total variation''' explained by the first k principal components. | |||
<li>The principal component of a random vector are not scale-invariant. For example, given three variables, say weight in pounds, height in feet, and age in years, we may seek a principal component expressed say in ounces, inches, and decades. Two procedures see feasible: | |||
* Multiple the data by 16, 12 and .1, respectively, and then carry out a PCA, | |||
* Carry out a PCA and then multiply the elements of the relevant component by 16, 12 and .1. | |||
Unfortunately the above procedures do not generally lead to the same result | |||
</li> | |||
<li>Number of principals? | |||
* Include just enough components to explain say 90% of the total variations '''scree graph'''; | |||
* Exclude those principal components whose eigenvalues are less than the average (Kaiser) | |||
<li>'''Rotation/loadings matrix is orthogonal''' | |||
<syntaxhighlight lang='r'> | |||
x <- matrix(c(10,6,12,5,11,4,9,7,8,5,10,6,3,3,2.5,2,2,2.8,1.3,4,1,1,2,7), | |||
nr=4) | |||
x <- t(x) # 6 samples, 4 genes | |||
rownames(x) <- paste("mouse",1:6) | |||
colnames(x) <- paste("gene", 1:4) | |||
pr <- prcomp(x, scale = T) | |||
t(pr$rotation) %*% pr$rotation - diag(4) | |||
# PC1 PC2 PC3 PC4 | |||
# PC1 0.000000e+00 0.000000e+00 1.804112e-16 -2.081668e-17 | |||
# PC2 0.000000e+00 -1.110223e-16 1.387779e-16 -2.220446e-16 | |||
# PC3 1.804112e-16 1.387779e-16 -2.220446e-16 -5.551115e-17 | |||
# PC4 -2.081668e-17 -2.220446e-16 -5.551115e-17 6.661338e-16 | |||
range(t(pr$rotation) %*% pr$rotation - diag(4)) | |||
# [1] -2.220446e-16 6.661338e-16 | |||
range(pr$rotation %*% t(pr$rotation) - diag(4)) | |||
[1] -6.661338e-16 2.220446e-16 | |||
</syntaxhighlight> | |||
<li>'''Principals are orthogonal''' | |||
<syntaxhighlight lang='r'> | |||
cor(pr$x) |> round(3) | |||
# PC1 PC2 PC3 PC4 | |||
# PC1 1 0 0 0 | |||
# PC2 0 1 0 0 | |||
# PC3 0 0 1 0 | |||
# PC4 0 0 0 1 | |||
</syntaxhighlight> | |||
<li>'''SLC''' (standardized linear combination). A linear combination <math>l' x</math> is SLC if <math>\sum l_i^2 = 1 </math>. | |||
<syntaxhighlight lang='r'> | |||
apply(pr$rotation, 2, function(x) sum(x^2)) | |||
# PC1 PC2 PC3 PC4 | |||
# 1 1 1 1 | |||
</syntaxhighlight> | |||
<li>No SLC of x has a variance larger than <math>\lambda_1</math>, the variance of the first principal component. | |||
<li>If <math>\alpha = a'x</math> is a SLC of x which is uncorrelated with the first k principal component of x, then the variance of <math>\alpha</math> is maximized when <math>\alpha</math> is the (k+1)th principal component of x. | |||
</li> | |||
</ul> | |||
== | = Tips = | ||
== Removing Zero Variance Columns == | |||
[https://www.ttested.com/removing-zero-variance-columns/ Efficiently Removing Zero Variance Columns (An Introduction to Benchmarking)] | |||
<pre> | <pre> | ||
# Assume df is a data frame or a list (matrix is not enough!) | |||
removeZeroVar3 <- function(df){ | |||
df[, !sapply(df, function(x) min(x) == max(x))] | |||
set. | } | ||
x <- | # Assume df is a matrix | ||
removeZeroVar3 <- function(df){ | |||
df[, !apply(df, 2, function(x) min(x) == max(x))] | |||
summary( | } | ||
# benchmark | |||
dim(t(tpmlog)) # [1] 58 28109 | |||
system.time({a <- t(tpmlog); a <- a[, apply(a, 2, sd) !=0]}) # 0.54 | |||
system.time({a <- t(tpmlog); a <- removeZeroVar3(a)}) # 0.18 | |||
</pre> | |||
[https://stackoverflow.com/a/15069056 Removal of constant columns in R] | |||
== Do not scale your matrix == | |||
https://privefl.github.io/blog/(Linear-Algebra)-Do-not-scale-your-matrix/ | |||
== Apply the transformation on test data == | |||
* [https://stackoverflow.com/a/42325998 How to use PCA on test set (code)] | |||
* [https://stats.stackexchange.com/a/72847 How to use R prcomp results for prediction?] | |||
* [https://ai.stackexchange.com/a/8684 How to perform PCA in the validation/test set?] | |||
== Calculated by Hand == | |||
http://strata.uga.edu/software/pdf/pcaTutorial.pdf | |||
== Variance explained by the first XX components == | |||
<ul> | |||
<li>[https://youtu.be/FgakZw6K1QQ?t=936 StatQuest: Principal Component Analysis (PCA), Step-by-Step]. So the variance explained by the K-th principals means (pr$sdev^2)[k]/sum(pr$sdev^2). | |||
<syntaxhighlight lang='r'> | |||
x <- matrix(c(10,6,12,5,11,4,9,7,8,5,10,6,3,3,2.5,2,2,2.8,1.3,4,1,1,2,7), nr=4) | |||
x <- t(x) # 6 samples, 4 genes | |||
rownames(x) <- paste("mouse",1:6) | |||
colnames(x) <- paste("gene", 1:4) | |||
pr <- prcomp(x, scale = T) | |||
names(pr) | |||
# [1] "sdev" "rotation" "center" "scale" "x" | |||
summary(pr) | |||
# Importance of components: | # Importance of components: | ||
# | # PC1 PC2 PC3 PC4 | ||
# Standard deviation 1. | # Standard deviation 1.6966 1.0091 0.29225 0.13321 | ||
# Proportion of Variance 0. | # Proportion of Variance 0.7197 0.2546 0.02135 0.00444 | ||
# Cumulative Proportion 0. | # Cumulative Proportion 0.7197 0.9742 0.99556 1.00000 | ||
apply(x, 2, var)|> sum() | |||
# [1] 48.10667 | |||
apply(pr$x, 2, var) |> sum() | |||
# [1] 4 | |||
cumsum(pr$sdev^2) | |||
# [1] 2.878596 3.896846 3.982256 4.000000 | |||
cumsum(pr$sdev^2)/sum(pr$sdev^2) | |||
# [1] 0.7196489 0.9742115 0.9955639 1.0000000 | |||
round(pr$sdev^2/sum(pr$sdev^2), 4) | |||
# [1] 0.7196 0.2546 0.0214 0.0044 | |||
pr$x # n x p | |||
# PC1 PC2 PC3 PC4 | |||
# mouse 1 -1.968265 -0.58969012 0.19246648 -0.04876951 | |||
# mouse 2 -1.350451 0.80465212 -0.48923602 0.04034721 | |||
# mouse 3 -1.268878 0.09892713 0.28501094 0.01538360 | |||
# mouse 4 1.368867 -1.36891596 -0.20417800 -0.13328931 | |||
# mouse 5 1.484377 -0.38237471 0.06095533 0.23453002 | |||
# mouse 6 1.734349 1.43740154 0.15498128 -0.10820202 | |||
pr$rotation | |||
# PC1 PC2 PC3 PC4 | |||
# gene 1 -0.5723917 0.01405298 -0.8132373 0.1039973 | |||
# gene 2 -0.5327490 -0.39598718 0.4450008 0.6011214 | |||
# gene 3 -0.5839377 0.00459261 0.3154255 -0.7479990 | |||
# gene 4 -0.2180896 0.91813701 0.2027959 0.2614100 | |||
pr$center | |||
# gene 1 gene 2 gene 3 gene 4 | |||
# 5.833333 3.633333 6.133333 5.166667 | |||
pr$scale | |||
# gene 1 gene 2 gene 3 gene 4 | |||
# 4.355074 1.768238 4.716637 1.940790 | |||
scale(x) %*% pr$rotation | |||
# PC1 PC2 PC3 PC4 | |||
# mouse 1 -1.968265 -0.58969012 0.19246648 -0.04876951 | |||
# mouse 2 -1.350451 0.80465212 -0.48923602 0.04034721 | |||
# mouse 3 -1.268878 0.09892713 0.28501094 0.01538360 | |||
# mouse 4 1.368867 -1.36891596 -0.20417800 -0.13328931 | |||
# mouse 5 1.484377 -0.38237471 0.06095533 0.23453002 | |||
# mouse 6 1.734349 1.43740154 0.15498128 -0.10820202 | |||
</syntaxhighlight> | |||
<li>[https://www.displayr.com/singular-value-decomposition-in-r/ Singular Value Decomposition (SVD) Tutorial Using Examples in R] | |||
<pre> | |||
summary( | svd2 <- svd(Z) | ||
variance.explained <- prop.table(svd2$d^2) | |||
</pre> | |||
OR | |||
<pre> | |||
pr <- prcomp(USArrests, scale. = TRUE) # PS default is scale. = FALSE | |||
summary(pr) | |||
# Importance of components: | # Importance of components: | ||
# PC1 PC2 | # PC1 PC2 PC3 PC4 | ||
# Standard deviation | # Standard deviation 1.5749 0.9949 0.59713 0.41645 | ||
# Proportion of Variance 0.6201 0.2474 0.08914 0.04336 <------- | |||
# Cumulative Proportion 0.6201 0.8675 0.95664 1.00000 | |||
# Proportion of Variance 0. | pr$sdev^2/ sum(pr$sdev^2) | ||
# [1] 0.62006039 0.24744129 0.08914080 0.04335752 | |||
</pre> | |||
</ul> | |||
= Visualization = | |||
<ul> | |||
<li>[https://www.biostars.org/p/461026/ Tutorial:Basic normalization, batch correction and visualization of RNA-seq data]. log(CPM) was used. | |||
<li>[https://support.bioconductor.org/p/98473/ DESeq2 - PCAplot differs between rlog and vsd transformation]. ''' VST''' is the one to use or [https://support.bioconductor.org/p/82556/ log2(count + 1)], which can be performed with normTransform(). | |||
<li>ggfortify or the basic method | |||
* [https://plotly.com/ggplot2/pca-visualization/ PCA Visualization in ggplot2] - ggplot2::autoplot(). [https://www.geeksforgeeks.org/how-to-make-pca-plot-with-r/ How To Make PCA Plot with R] | |||
* [https://stackoverflow.com/a/71221558 Difference ggplot2 and autoplot() after prcomp?] In the autoplot method, the principal components are '''scaled'''. We can turn off the scaling by specifying "scale =0" in autoplot(). | |||
* Add ellipse - [https://stackoverflow.com/questions/71221048/difference-ggplot2-and-autoplot-after-prcomp Difference ggplot2 and autoplot() after prcomp?] (same post as above, use the basic method instead of autoplot()). We can also use '''factoextra''' package to draw a PCA plot with ellipses. [https://statisticsglobe.com/draw-ellipse-plot-groups-pca-r Draw Ellipse Plot for Groups in PCA in R (2 Examples)]. | |||
[[File:Pca autoplot.png|250px]] [[File:Pca directly2.png|250px]] [[File:Pca factoextra.png|250px]] [[File:Pca ggplot2.png|250px]] </BR> | |||
[https://rdocumentation.org/packages/ggfortify/versions/0.4.15/topics/autoplot.pca_common ?ggfortify::autoplot.prcomp] | |||
<pre> | |||
library(ggfortify) | |||
iris[1:2,] | |||
# Sepal.Length Sepal.Width Petal.Length Petal.Width Species | |||
# 1 5.1 3.5 1.4 0.2 setosa | |||
# 2 4.9 3.0 1.4 0.2 setosa | |||
df <- iris[1:4] # exclude "Species" column | |||
pca_res <- prcomp(df, scale. = TRUE) | |||
p <- autoplot(pca_res, data = iris, colour = 'Species') | |||
# OR turn off scaling on axes in order to get the same plot as the direct method | |||
# p <- autoplot(pca_res, data = iris, colour = 'Species', scale = 0) | |||
p # PC1 (72.96%), PC2 (22.85%) | |||
library(plotly) | |||
ggplotly(p) | |||
</pre> | |||
Basic method | |||
<pre> | |||
group <- as.numeric(iris$Species) | |||
# | # ggplot2's palette | ||
gg_color_hue <- function(n) { | |||
hues = seq(15, 375, length = n + 1) | |||
hcl(h = hues, l = 65, c = 100)[1:n] | |||
} | |||
n <- 3 # iris$Species has 3 levels | |||
cols = gg_color_hue(n) | |||
# | |||
prp <- pca_res$sdev^2/ sum(pca_res$sdev^2) | |||
# | xlab <- paste0("PC1 (", round(prp[1]*100,2), "%)") | ||
ylab <- paste0("PC2 (", round(prp[2]*100,2), "%)") | |||
omar <- par(mar=c(5,4,4,8)) | |||
plot(pca_res$x[, 1], pca_res$x[, 2], | |||
xlab = xlab, ylab = ylab, | |||
col = cols[group], pch=16) | |||
grid(NULL, NULL, lty=3, lwd=1, col="#000000") | |||
par(xpd=TRUE) | |||
legend(4, 1, legend = levels(iris$Species), col=cols, pch=16, bty = "n", title = "Species") | |||
par(xpd=FALSE) | |||
par(omar) | |||
</pre> | |||
[https://rpkgs.datanovia.com/factoextra/reference/fviz_pca.html ? factoextra::fviz_pca_ind] | |||
<pre> | |||
library(factoextra) | |||
fviz_pca_ind(pca_res, | |||
habillage=iris$Species, | |||
label = "none", | |||
ellipse.level=0.95, # default | |||
addEllipses=TRUE, title = "PCA") | |||
</pre> | </pre> | ||
<li>Why the ellipses generated by fviz_pca_ind() and stat_ellipse() are different? [https://stackoverflow.com/a/69860285 How does R calculate the PCA ellipses?] | |||
* [https://ggplot2.tidyverse.org/reference/stat_ellipse.html Compute normal data ellipses]. Default is using the t-distribution. | |||
* [https://support.bioconductor.org/p/9159343/ Plot PCA with ellipses using ggplot] | |||
* [https://stackoverflow.com/a/63645455 Error: Too few points to calculate an ellipse with 3 points? - R] factoextra + ggforce packages | |||
* Compute and draw an ellipse using base R functions. type ="norm" case. | |||
<pre> | |||
# Example dataset | |||
data <- iris | |||
# ggplot2 method | |||
ggplot(data, aes(x = Sepal.Length, y = Sepal.Width)) + | |||
geom_point() + | |||
stat_ellipse(level = 0.95, type = "norm") + | |||
ggtitle("norm distribution") | |||
# Base R method | |||
# Select x and y variables | |||
x <- data$Sepal.Length | |||
y <- data$Sepal.Width | |||
# Calculate the mean of x and y | |||
mean_x <- mean(x) | |||
mean_y <- mean(y) | |||
# Create a covariance matrix | |||
cov_matrix <- cov(cbind(x, y)) | |||
# Calculate the eigenvalues and eigenvectors of the covariance matrix | |||
eigen_decomp <- eigen(cov_matrix) | |||
eig_val <- eigen_decomp$values | |||
eig_vec <- eigen_decomp$vectors | |||
# Set the confidence level | |||
confidence_level <- 0.95 | |||
# Get the number of observations | |||
n <- length(x) | |||
# Compute the scaling factor | |||
# | t_val <- sqrt(2 * qf(confidence_level, 2, n - 1)) | ||
= | # Generate points for the ellipse | ||
theta <- seq(0, 2 * pi, length.out = 100) | |||
ellipse_coords <- t(eig_vec %*% diag(sqrt(eig_val)) %*% rbind(cos(theta), sin(theta))) * t_val | |||
# Translate the ellipse to the mean | |||
ellipse_coords[,1] <- ellipse_coords[,1] + mean_x | |||
ellipse_coords[,2] <- ellipse_coords[,2] + mean_y | |||
= | # Plot the points and the ellipse | ||
plot(x, y, xlab = "Sepal Length", ylab = "Sepal Width", main = "Scatter Plot with Confidence Ellipse") | |||
lines(ellipse_coords, col = "red", lwd = 2) | |||
</pre> | |||
<li>[https://stackoverflow.com/a/61157732 Two categorical variables: one point shape and one fill color?] | |||
<li>[http://oracledmt.blogspot.com/2007/06/way-cooler-pca-and-visualization-linear.html PCA and Visualization] | |||
<li>Scree plots from the [http://www.sthda.com/english/wiki/factominer-and-factoextra-principal-component-analysis-visualization-r-software-and-data-mining FactoMineR] package (based on ggplot2) | |||
<li>[https://twitter.com/mikelove/status/1513468603806453761?s=20&t=H79gmvzaqRTtSp11o8kxmA 2 lines of code], [https://rdrr.io/bioc/DESeq2/man/plotPCA.html plotPCA()] from DESeq2 | |||
</ul> | |||
== Interactive Principal Component Analysis == | == Interactive Principal Component Analysis == | ||
[https://www.business-science.io/code-tools/2020/12/15/pca.html Interactive Principal Component Analysis in R] | * Use the [[R#Identify.2FLocate_Points_in_a_Scatter_Plot|identify()]] function and mouse clicks to label some points (also return the indices of these points). | ||
* [https://www.business-science.io/code-tools/2020/12/15/pca.html Interactive Principal Component Analysis in R] | |||
= What does it do if we choose center=FALSE in prcomp()? = | = center and scale = | ||
== What does it do if we choose center=FALSE in prcomp()? == | |||
In USArrests data, use center=FALSE gives a better scatter plot of the first 2 PCA components. | In USArrests data, use center=FALSE gives a better scatter plot of the first 2 PCA components. | ||
| Line 176: | Line 416: | ||
</pre> | </pre> | ||
= "scale. = TRUE" and Mean subtraction = | == "scale. = TRUE" and Mean subtraction == | ||
<ul> | |||
<li>PCA is sensitive to the scaling of the variables. See [https://en.wikipedia.org/wiki/Principal_component_analysis#Further_considerations PCA -> Further considerations]. | |||
<li>https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/prcomp | |||
<li>By default '''scale. = FALSE''' in prcomp() | |||
<li>By default, it centers the variable to have mean equals to zero. With parameter scale. = T, we normalize the variables to have standard deviation equals to 1. See also [https://stats.stackexchange.com/a/268268 Normalizing all the variarbles vs. using scale=TRUE option in prcomp in R]. | |||
<li>[https://www.analyticsvidhya.com/blog/2016/03/practical-guide-principal-component-analysis-python/ Practical Guide to Principal Component Analysis (PCA) in R & Python] | |||
<li>[https://www.researchgate.net/post/What-is-the-best-way-to-scale-parameters-before-running-a-Principal-Component-Analysis-PCA What is the best way to scale parameters before running a Principal Component Analysis (PCA)?] | |||
* Let's compare the difference | * As a rule of thumb, if all your variables are measured on the same scale and have the same unit, it might be a good idea *not* to scale the variables (i.e., PCA based on the covariance matrix). '''If you want to maximize variation, it is fair to let variables with more variation contribute more.''' On the other hand, If you have different types of variables with different units, it is probably wise to scale the data first (i.e., PCA based on the correlation matrix). | ||
* If all your variables are recorded in the same scale and/or the difference in variable magnitudes are of interest, you may choose not to normalize your data prior to PCA. See [https://stats.stackexchange.com/a/268268 Normalizing all the variarbles vs. using scale=TRUE option in prcomp in R] | |||
<li>Let's compare the difference using the [https://stats.stackexchange.com/questions/69157/why-do-we-need-to-normalize-data-before-principal-component-analysis-pca USArrests] data | |||
<pre> | <pre> | ||
USArrests[1:3,] | USArrests[1:3,] | ||
| Line 233: | Line 475: | ||
# u (or v) are also different | # u (or v) are also different | ||
</pre> | </pre> | ||
<li>[https://stats.stackexchange.com/questions/53/pca-on-correlation-or-covariance Biplot] | |||
</ul> | |||
= Number of components = | |||
[https://statisticaloddsandends.wordpress.com/2018/10/15/obtaining-the-number-of-components-from-cross-validation-of-principal-components-regression/ Obtaining the number of components from cross validation of principal components regression] | |||
== AIC/BIC in estimating the number of components == | |||
[https://projecteuclid.org/euclid.aos/1525313075 Consistency of AIC and BIC in estimating the number of significant components in high-dimensional principal component analysis] | |||
= PCA and SVD = | |||
Using the SVD to perform PCA makes much better sense numerically than forming the covariance matrix to begin with, since the formation of <math>X X^T</math> can cause loss of precision. | |||
* http://math.stackexchange.com/questions/3869/what-is-the-intuitive-relationship-between-svd-and-pca | |||
* [https://stats.stackexchange.com/a/134283 Relationship between SVD and PCA. How to use SVD to perform PCA?] | |||
= | == R example == | ||
<pre> | <pre> | ||
covMat <- matrix(c(4, 0, 0, 1), nr=2) | |||
p <- 2 | |||
n <- 1000 | |||
set.seed(1) | |||
x <- mvtnorm::rmvnorm(n, rep(0, p), covMat) | |||
svdx <- svd(x) | |||
result= prcomp(x, scale = TRUE) | |||
summary(result) | |||
# Importance of components: | |||
# PC1 PC2 | |||
# Standard deviation 1.0004 0.9996 | |||
# Proportion of Variance 0.5004 0.4996 | |||
# Cumulative Proportion 0.5004 1.0000 | |||
# It seems scale = FALSE result can reflect the original data | |||
result2 <- prcomp(x, scale = FALSE) # DEFAULT | |||
summary(result2) | |||
# Importance of components: | |||
# PC1 PC2 | |||
# Standard deviation 2.1332 1.0065 ==> Close to the original data | |||
# ==> How to compute | |||
# sqrt(eigen(var(x))$values ) = 2.133203 1.006460 | |||
# Proportion of Variance 0.8179 0.1821 ==> How to verify | |||
# 2.1332^2/(2.1332^2+1.0065^2) = 0.8179 | |||
# Cumulative Proportion 0.8179 1.0000 | |||
result2$sdev | |||
# 2.133203 1.006460 # sqrt( SS(distance)/(n-1) ) | |||
result2$sdev^2 / sum(result2$sdev^2) | |||
# 0.8179279 0.1820721 | |||
result2$rotation | |||
# PC1 PC2 | |||
# [1,] 0.9999998857 -0.0004780211 | |||
# [2,] -0.0004780211 -0.9999998857 | |||
result2$sdev^2 * (n-1) | |||
# [1] 4546.004 1011.948 # SS(distance) | |||
# eigenvalue for PC1 = singular value^2 | |||
svd(scale(x, center=T, scale=F))$d | |||
# [1] 67.42406 31.81113 | |||
svd(scale(x, center=T, scale=F))$d ^ 2 # SS(distance) | |||
# [1] 4546.004 1011.948 | |||
svd(scale(x, center=T, scale=F))$d / sqrt(nrow(x) -1) | |||
# [1] 2.133203 1.006460 ==> This is the same as prcomp()$sdev | |||
svd(scale(x, center=F, scale=F))$d / sqrt(nrow(x) -1) | |||
# [1] 2.135166 1.006617 ==> So it does not matter to center or scale | |||
svd(var(x))$d | |||
# [1] 4.550554 1.012961 | |||
svd(scale(x, center=T, scale=F))$v # same as result2$rotation | |||
# [,1] [,2] | |||
# [1,] 0.9999998857 -0.0004780211 | |||
# [2,] -0.0004780211 -0.9999998857 | |||
sqrt(eigen(var(x))$values ) | |||
# [1] 2.133203 1.006460 | |||
eigen(t(scale(x,T,F)) %*% scale(x,T,F))$values # SS(distance) | |||
# [1] 4546.004 1011.948 | |||
sqrt(eigen(t(scale(x,T,F)) %*% scale(x,T,F))$values ) # Same as svd(scale(x.T,F))$d | |||
# [1] 67.42406 31.81113. | |||
# So SS(distance) = eigen(t(scale(x,T,F)) %*% scale(x,T,F))$values | |||
# = svd(scale(x.T,F))$d ^ 2 | |||
</pre> | </pre> | ||
= | = Related to Factor Analysis = | ||
* http://www.aaronschlegel.com/factor-analysis-introduction-principal-component-method-r/. | |||
* http://support.minitab.com/en-us/minitab/17/topic-library/modeling-statistics/multivariate/principal-components-and-factor-analysis/differences-between-pca-and-factor-analysis/ | |||
In short, | |||
# In Principal Components Analysis, the components are calculated as linear combinations of the original variables. In Factor Analysis, the original variables are defined as linear combinations of the factors. | |||
# In Principal Components Analysis, the goal is to explain as much of the total variance in the variables as possible. The goal in Factor Analysis is to explain the covariances or correlations between the variables. | |||
# Use Principal Components Analysis to reduce the data into a smaller number of components. Use Factor Analysis to understand what constructs underlie the data. | |||
= prcomp vs princomp = | = prcomp vs princomp = | ||
[http://www.sthda.com/english/articles/31-principal-component-methods-in-r-practical-guide/118-principal-component-analysis-in-r-prcomp-vs-princomp/ prcomp vs princomp] from sthda. '''prcomp'''() is preferred compared to princomp(). | [http://www.sthda.com/english/articles/31-principal-component-methods-in-r-practical-guide/118-principal-component-analysis-in-r-prcomp-vs-princomp/ prcomp vs princomp] from sthda. '''prcomp'''() is preferred compared to princomp(). | ||
= Missing data = | |||
[https://bioconductor.org/packages/release/bioc/html/pcaMethods.html pcaMethods] | |||
= Relation to [http://en.wikipedia.org/wiki/Multidimensional_scaling Multidimensional scaling/MDS] = | = Relation to [http://en.wikipedia.org/wiki/Multidimensional_scaling Multidimensional scaling/MDS] = | ||
| Line 276: | Line 582: | ||
= Matrix factorization methods = | = Matrix factorization methods = | ||
http://joelcadwell.blogspot.com/2015/08/matrix-factorization-comes-in-many.html Review of principal component analysis (PCA), K-means clustering, nonnegative matrix factorization (NMF) and archetypal analysis (AA). | http://joelcadwell.blogspot.com/2015/08/matrix-factorization-comes-in-many.html Review of principal component analysis (PCA), K-means clustering, nonnegative matrix factorization (NMF) and archetypal analysis (AA). | ||
= Outlier samples = | = Outlier samples = | ||
| Line 285: | Line 588: | ||
== Reconstructing images == | == Reconstructing images == | ||
[https://kieranhealy.org/blog/archives/2019/10/27/reconstructing-images-using-pca/ Reconstructing images using PCA] | [https://kieranhealy.org/blog/archives/2019/10/27/reconstructing-images-using-pca/ Reconstructing images using PCA] | ||
= Sparse PCA = | |||
* https://en.wikipedia.org/wiki/Sparse_PCA | |||
* [https://hbiostat.org/rmsc/impred.html#sec-impred-sparsepc 8.6.1 Sparse Principal Components] from Harrell's "Regression Modeling Strategies" book | |||
* [https://cran.r-project.org/web/packages/pcaPP/index.html pcaPP] package | |||
= Misc = | = Misc = | ||
[https://doi.org/10.1111/biom.13159 Data reduction prior to inference: Are there consequences of comparing groups using a t‐test based on principal component scores?] Bedrick 2019 | [https://doi.org/10.1111/biom.13159 Data reduction prior to inference: Are there consequences of comparing groups using a t‐test based on principal component scores?] Bedrick 2019 | ||
= PCA for Categorical Variables = | |||
* [http://sthda.com/english/articles/31-principal-component-methods-in-r-practical-guide/115-famd-factor-analysis-of-mixed-data-in-r-essentials/ FAMD - Factor Analysis of Mixed Data in R: Essentials] | |||
* [https://finnstats.com/index.php/2022/11/20/pca-for-categorical-variables-in-r/ PCA for Categorical Variables in R]. Factorial Analysis of Mixed Data (FAMD) Is a PCA for Categorical Variables Alternate. | |||
= psych package = | = psych package = | ||
Latest revision as of 22:51, 8 September 2024
Principal component analysis
What is PCA
- https://en.wikipedia.org/wiki/Principal_component_analysis
- StatQuest: Principal Component Analysis (PCA), Step-by-Step (video)
- StatQuest: PCA in R
- Using R for Multivariate Analysis
- How exactly does PCA work? What PCA (with SVD) does is, it finds the best fit line for these data points which minimizes the distance between the data points and their projections on the best fit line.
- What Is Principal Component Analysis (PCA) and How It Is Used? PCA creates a visualization of data that minimizes residual variance in the least squares sense and maximizes the variance of the projection coordinates.
- Principal Component Analysis: a brief intro for biologists
Linear Algebra
Linear Algebra for Data Science with examples in R
R source code
> stats:::prcomp.default
function (x, retx = TRUE, center = TRUE, scale. = FALSE, tol = NULL,
...)
{
x <- as.matrix(x)
x <- scale(x, center = center, scale = scale.)
cen <- attr(x, "scaled:center")
sc <- attr(x, "scaled:scale")
if (any(sc == 0))
stop("cannot rescale a constant/zero column to unit variance")
s <- svd(x, nu = 0)
s$d <- s$d/sqrt(max(1, nrow(x) - 1))
if (!is.null(tol)) {
rank <- sum(s$d > (s$d[1L] * tol))
if (rank < ncol(x)) {
s$v <- s$v[, 1L:rank, drop = FALSE]
s$d <- s$d[1L:rank]
}
}
dimnames(s$v) <- list(colnames(x), paste0("PC", seq_len(ncol(s$v))))
r <- list(sdev = s$d, rotation = s$v, center = if (is.null(cen)) FALSE else cen,
scale = if (is.null(sc)) FALSE else sc)
if (retx)
r$x <- x %*% s$v
class(r) <- "prcomp"
r
}
<bytecode: 0x000000003296c7d8>
<environment: namespace:stats>
rank of a matrix
R example
Principal component analysis (PCA) in R including bi-plot.
R built-in plot
http://genomicsclass.github.io/book/pages/pca_svd.html
pc <- prcomp(x) group <- as.numeric(tab$Tissue) plot(pc$x[, 1], pc$x[, 2], col = group, main = "PCA", xlab = "PC1", ylab = "PC2")
The meaning of colors can be found by palette().
- black
- red
- green3
- blue
- cyan
- magenta
- yellow
- gray
palmerpenguins data
Theory with an example
Principal Component Analysis in R: prcomp vs princomp
Biplot
- Biplots are everywhere: where do they come from?
- ggbiplot. Principal Component Analysis in R Tutorial from datacamp.
- Exploring Multivariate Data with Principal Component Analysis (PCA) Biplot in R
factoextra
- https://cran.r-project.org/web/packages/factoextra/index.html
- Factoextra R Package: Easy Multivariate Data Analyses and Elegant Visualization
Properties
- Definition. If x is a random vector with mean [math]\displaystyle{ \mu }[/math] and covariance matrix [math]\displaystyle{ \Sigma }[/math], the the principal component transformation is
- [math]\displaystyle{ y = \Gamma ' (x - \mu), }[/math]
- Properties
- [math]\displaystyle{ E(y_i) =0, }[/math]
- [math]\displaystyle{ Var(y_i) =\lambda_i, }[/math]
- [math]\displaystyle{ Cov(y_i, y_j) =0, i \neq j, }[/math]
- [math]\displaystyle{ Var(y_1) \geq Var(y_2) \geq \cdots Var(y_p) \geq 0, }[/math]
- [math]\displaystyle{ \sum Var(y_i) = tr \Sigma, }[/math] total variation
- [math]\displaystyle{ \prod Var(y_i) = |\Sigma| }[/math].
- The sum of the first k eigenvalues divided by the sum of all the eigenvalues, [math]\displaystyle{ (\lambda_1 + \cdots + \lambda_k)/(\lambda_1 + \cdots + \lambda_p) }[/math], represents the proportion of total variation explained by the first k principal components.
- The principal component of a random vector are not scale-invariant. For example, given three variables, say weight in pounds, height in feet, and age in years, we may seek a principal component expressed say in ounces, inches, and decades. Two procedures see feasible:
- Multiple the data by 16, 12 and .1, respectively, and then carry out a PCA,
- Carry out a PCA and then multiply the elements of the relevant component by 16, 12 and .1.
- Number of principals?
- Include just enough components to explain say 90% of the total variations scree graph;
- Exclude those principal components whose eigenvalues are less than the average (Kaiser)
- Rotation/loadings matrix is orthogonal
x <- matrix(c(10,6,12,5,11,4,9,7,8,5,10,6,3,3,2.5,2,2,2.8,1.3,4,1,1,2,7), nr=4) x <- t(x) # 6 samples, 4 genes rownames(x) <- paste("mouse",1:6) colnames(x) <- paste("gene", 1:4) pr <- prcomp(x, scale = T) t(pr$rotation) %*% pr$rotation - diag(4) # PC1 PC2 PC3 PC4 # PC1 0.000000e+00 0.000000e+00 1.804112e-16 -2.081668e-17 # PC2 0.000000e+00 -1.110223e-16 1.387779e-16 -2.220446e-16 # PC3 1.804112e-16 1.387779e-16 -2.220446e-16 -5.551115e-17 # PC4 -2.081668e-17 -2.220446e-16 -5.551115e-17 6.661338e-16 range(t(pr$rotation) %*% pr$rotation - diag(4)) # [1] -2.220446e-16 6.661338e-16 range(pr$rotation %*% t(pr$rotation) - diag(4)) [1] -6.661338e-16 2.220446e-16 - Principals are orthogonal
cor(pr$x) |> round(3) # PC1 PC2 PC3 PC4 # PC1 1 0 0 0 # PC2 0 1 0 0 # PC3 0 0 1 0 # PC4 0 0 0 1
- SLC (standardized linear combination). A linear combination [math]\displaystyle{ l' x }[/math] is SLC if [math]\displaystyle{ \sum l_i^2 = 1 }[/math].
apply(pr$rotation, 2, function(x) sum(x^2)) # PC1 PC2 PC3 PC4 # 1 1 1 1
- No SLC of x has a variance larger than [math]\displaystyle{ \lambda_1 }[/math], the variance of the first principal component.
- If [math]\displaystyle{ \alpha = a'x }[/math] is a SLC of x which is uncorrelated with the first k principal component of x, then the variance of [math]\displaystyle{ \alpha }[/math] is maximized when [math]\displaystyle{ \alpha }[/math] is the (k+1)th principal component of x.
Tips
Removing Zero Variance Columns
Efficiently Removing Zero Variance Columns (An Introduction to Benchmarking)
# Assume df is a data frame or a list (matrix is not enough!)
removeZeroVar3 <- function(df){
df[, !sapply(df, function(x) min(x) == max(x))]
}
# Assume df is a matrix
removeZeroVar3 <- function(df){
df[, !apply(df, 2, function(x) min(x) == max(x))]
}
# benchmark
dim(t(tpmlog)) # [1] 58 28109
system.time({a <- t(tpmlog); a <- a[, apply(a, 2, sd) !=0]}) # 0.54
system.time({a <- t(tpmlog); a <- removeZeroVar3(a)}) # 0.18
Removal of constant columns in R
Do not scale your matrix
https://privefl.github.io/blog/(Linear-Algebra)-Do-not-scale-your-matrix/
Apply the transformation on test data
- How to use PCA on test set (code)
- How to use R prcomp results for prediction?
- How to perform PCA in the validation/test set?
Calculated by Hand
http://strata.uga.edu/software/pdf/pcaTutorial.pdf
Variance explained by the first XX components
- StatQuest: Principal Component Analysis (PCA), Step-by-Step. So the variance explained by the K-th principals means (pr$sdev^2)[k]/sum(pr$sdev^2).
x <- matrix(c(10,6,12,5,11,4,9,7,8,5,10,6,3,3,2.5,2,2,2.8,1.3,4,1,1,2,7), nr=4) x <- t(x) # 6 samples, 4 genes rownames(x) <- paste("mouse",1:6) colnames(x) <- paste("gene", 1:4) pr <- prcomp(x, scale = T) names(pr) # [1] "sdev" "rotation" "center" "scale" "x" summary(pr) # Importance of components: # PC1 PC2 PC3 PC4 # Standard deviation 1.6966 1.0091 0.29225 0.13321 # Proportion of Variance 0.7197 0.2546 0.02135 0.00444 # Cumulative Proportion 0.7197 0.9742 0.99556 1.00000 apply(x, 2, var)|> sum() # [1] 48.10667 apply(pr$x, 2, var) |> sum() # [1] 4 cumsum(pr$sdev^2) # [1] 2.878596 3.896846 3.982256 4.000000 cumsum(pr$sdev^2)/sum(pr$sdev^2) # [1] 0.7196489 0.9742115 0.9955639 1.0000000 round(pr$sdev^2/sum(pr$sdev^2), 4) # [1] 0.7196 0.2546 0.0214 0.0044 pr$x # n x p # PC1 PC2 PC3 PC4 # mouse 1 -1.968265 -0.58969012 0.19246648 -0.04876951 # mouse 2 -1.350451 0.80465212 -0.48923602 0.04034721 # mouse 3 -1.268878 0.09892713 0.28501094 0.01538360 # mouse 4 1.368867 -1.36891596 -0.20417800 -0.13328931 # mouse 5 1.484377 -0.38237471 0.06095533 0.23453002 # mouse 6 1.734349 1.43740154 0.15498128 -0.10820202 pr$rotation # PC1 PC2 PC3 PC4 # gene 1 -0.5723917 0.01405298 -0.8132373 0.1039973 # gene 2 -0.5327490 -0.39598718 0.4450008 0.6011214 # gene 3 -0.5839377 0.00459261 0.3154255 -0.7479990 # gene 4 -0.2180896 0.91813701 0.2027959 0.2614100 pr$center # gene 1 gene 2 gene 3 gene 4 # 5.833333 3.633333 6.133333 5.166667 pr$scale # gene 1 gene 2 gene 3 gene 4 # 4.355074 1.768238 4.716637 1.940790 scale(x) %*% pr$rotation # PC1 PC2 PC3 PC4 # mouse 1 -1.968265 -0.58969012 0.19246648 -0.04876951 # mouse 2 -1.350451 0.80465212 -0.48923602 0.04034721 # mouse 3 -1.268878 0.09892713 0.28501094 0.01538360 # mouse 4 1.368867 -1.36891596 -0.20417800 -0.13328931 # mouse 5 1.484377 -0.38237471 0.06095533 0.23453002 # mouse 6 1.734349 1.43740154 0.15498128 -0.10820202 - Singular Value Decomposition (SVD) Tutorial Using Examples in R
svd2 <- svd(Z) variance.explained <- prop.table(svd2$d^2)
OR
pr <- prcomp(USArrests, scale. = TRUE) # PS default is scale. = FALSE summary(pr) # Importance of components: # PC1 PC2 PC3 PC4 # Standard deviation 1.5749 0.9949 0.59713 0.41645 # Proportion of Variance 0.6201 0.2474 0.08914 0.04336 <------- # Cumulative Proportion 0.6201 0.8675 0.95664 1.00000 pr$sdev^2/ sum(pr$sdev^2) # [1] 0.62006039 0.24744129 0.08914080 0.04335752
Visualization
- Tutorial:Basic normalization, batch correction and visualization of RNA-seq data. log(CPM) was used.
- DESeq2 - PCAplot differs between rlog and vsd transformation. VST is the one to use or log2(count + 1), which can be performed with normTransform().
- ggfortify or the basic method
- PCA Visualization in ggplot2 - ggplot2::autoplot(). How To Make PCA Plot with R
- Difference ggplot2 and autoplot() after prcomp? In the autoplot method, the principal components are scaled. We can turn off the scaling by specifying "scale =0" in autoplot().
- Add ellipse - Difference ggplot2 and autoplot() after prcomp? (same post as above, use the basic method instead of autoplot()). We can also use factoextra package to draw a PCA plot with ellipses. Draw Ellipse Plot for Groups in PCA in R (2 Examples).
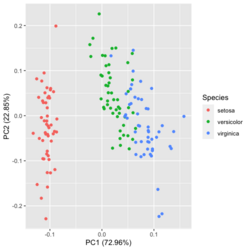
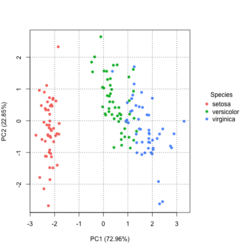
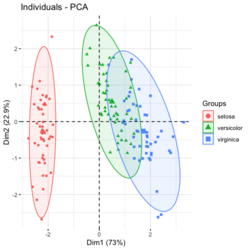
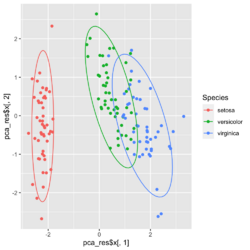
?ggfortify::autoplot.prcomplibrary(ggfortify) iris[1:2,] # Sepal.Length Sepal.Width Petal.Length Petal.Width Species # 1 5.1 3.5 1.4 0.2 setosa # 2 4.9 3.0 1.4 0.2 setosa df <- iris[1:4] # exclude "Species" column pca_res <- prcomp(df, scale. = TRUE) p <- autoplot(pca_res, data = iris, colour = 'Species') # OR turn off scaling on axes in order to get the same plot as the direct method # p <- autoplot(pca_res, data = iris, colour = 'Species', scale = 0) p # PC1 (72.96%), PC2 (22.85%) library(plotly) ggplotly(p)
Basic method
group <- as.numeric(iris$Species) # ggplot2's palette gg_color_hue <- function(n) { hues = seq(15, 375, length = n + 1) hcl(h = hues, l = 65, c = 100)[1:n] } n <- 3 # iris$Species has 3 levels cols = gg_color_hue(n) prp <- pca_res$sdev^2/ sum(pca_res$sdev^2) xlab <- paste0("PC1 (", round(prp[1]*100,2), "%)") ylab <- paste0("PC2 (", round(prp[2]*100,2), "%)") omar <- par(mar=c(5,4,4,8)) plot(pca_res$x[, 1], pca_res$x[, 2], xlab = xlab, ylab = ylab, col = cols[group], pch=16) grid(NULL, NULL, lty=3, lwd=1, col="#000000") par(xpd=TRUE) legend(4, 1, legend = levels(iris$Species), col=cols, pch=16, bty = "n", title = "Species") par(xpd=FALSE) par(omar)library(factoextra) fviz_pca_ind(pca_res, habillage=iris$Species, label = "none", ellipse.level=0.95, # default addEllipses=TRUE, title = "PCA") - Why the ellipses generated by fviz_pca_ind() and stat_ellipse() are different? How does R calculate the PCA ellipses?
- Compute normal data ellipses. Default is using the t-distribution.
- Plot PCA with ellipses using ggplot
- Error: Too few points to calculate an ellipse with 3 points? - R factoextra + ggforce packages
- Compute and draw an ellipse using base R functions. type ="norm" case.
# Example dataset data <- iris # ggplot2 method ggplot(data, aes(x = Sepal.Length, y = Sepal.Width)) + geom_point() + stat_ellipse(level = 0.95, type = "norm") + ggtitle("norm distribution") # Base R method # Select x and y variables x <- data$Sepal.Length y <- data$Sepal.Width # Calculate the mean of x and y mean_x <- mean(x) mean_y <- mean(y) # Create a covariance matrix cov_matrix <- cov(cbind(x, y)) # Calculate the eigenvalues and eigenvectors of the covariance matrix eigen_decomp <- eigen(cov_matrix) eig_val <- eigen_decomp$values eig_vec <- eigen_decomp$vectors # Set the confidence level confidence_level <- 0.95 # Get the number of observations n <- length(x) # Compute the scaling factor t_val <- sqrt(2 * qf(confidence_level, 2, n - 1)) # Generate points for the ellipse theta <- seq(0, 2 * pi, length.out = 100) ellipse_coords <- t(eig_vec %*% diag(sqrt(eig_val)) %*% rbind(cos(theta), sin(theta))) * t_val # Translate the ellipse to the mean ellipse_coords[,1] <- ellipse_coords[,1] + mean_x ellipse_coords[,2] <- ellipse_coords[,2] + mean_y # Plot the points and the ellipse plot(x, y, xlab = "Sepal Length", ylab = "Sepal Width", main = "Scatter Plot with Confidence Ellipse") lines(ellipse_coords, col = "red", lwd = 2) - Two categorical variables: one point shape and one fill color?
- PCA and Visualization
- Scree plots from the FactoMineR package (based on ggplot2)
- 2 lines of code, plotPCA() from DESeq2
Interactive Principal Component Analysis
- Use the identify() function and mouse clicks to label some points (also return the indices of these points).
- Interactive Principal Component Analysis in R
center and scale
What does it do if we choose center=FALSE in prcomp()?
In USArrests data, use center=FALSE gives a better scatter plot of the first 2 PCA components.
x1 = prcomp(USArrests) x2 = prcomp(USArrests, center=F) plot(x1$x[,1], x1$x[,2]) # looks random windows(); plot(x2$x[,1], x2$x[,2]) # looks good in some sense
"scale. = TRUE" and Mean subtraction
- PCA is sensitive to the scaling of the variables. See PCA -> Further considerations.
- https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/prcomp
- By default scale. = FALSE in prcomp()
- By default, it centers the variable to have mean equals to zero. With parameter scale. = T, we normalize the variables to have standard deviation equals to 1. See also Normalizing all the variarbles vs. using scale=TRUE option in prcomp in R.
- Practical Guide to Principal Component Analysis (PCA) in R & Python
- What is the best way to scale parameters before running a Principal Component Analysis (PCA)?
- As a rule of thumb, if all your variables are measured on the same scale and have the same unit, it might be a good idea *not* to scale the variables (i.e., PCA based on the covariance matrix). If you want to maximize variation, it is fair to let variables with more variation contribute more. On the other hand, If you have different types of variables with different units, it is probably wise to scale the data first (i.e., PCA based on the correlation matrix).
- If all your variables are recorded in the same scale and/or the difference in variable magnitudes are of interest, you may choose not to normalize your data prior to PCA. See Normalizing all the variarbles vs. using scale=TRUE option in prcomp in R
- Let's compare the difference using the USArrests data
USArrests[1:3,] # Murder Assault UrbanPop Rape # Alabama 13.2 236 58 21.2 # Alaska 10.0 263 48 44.5 # Arizona 8.1 294 80 31.0 pca1 <- prcomp(USArrests) # inappropriate, default is scale. = FALSE pca2 <- prcomp(USArrests, scale. = TRUE) pca1$x[1:3, 1:3] # PC1 PC2 PC3 # Alabama 64.80216 -11.448007 -2.494933 # Alaska 92.82745 -17.982943 20.126575 # Arizona 124.06822 8.830403 -1.687448 pca2$x[1:3, 1:3] # PC1 PC2 PC3 # Alabama -0.9756604 1.1220012 -0.43980366 # Alaska -1.9305379 1.0624269 2.01950027 # Arizona -1.7454429 -0.7384595 0.05423025 set.seed(1) X <- matrix(rnorm(10*100), nr=10) pca1 <- prcomp(X) pca2 <- prcomp(X, scale. = TRUE) range(abs(pca1$x - pca2$x)) # [1] 3.764350e-16 1.139182e+01 par(mfrow=c(1,2)) plot(pca1$x[,1], pca1$x[, 2], main='scale=F') plot(pca2$x[,1], pca2$x[, 2], main='scale=T') par(mfrow=c(1,1)) # rotation matrices are different # sdev are different # rotation matrices are different # Same observations for a long matrix too set.seed(1) X <- matrix(rnorm(10*100), nr=100) pca1 <- prcomp(X) pca2 <- prcomp(X, scale. = TRUE) range(abs(pca1$x - pca2$x)) # [1] 0.001915974 5.112158589 svd1 <- svd(USArrests) svd2 <- svd(scale(USArrests, F, T)) svd1$d # [1] 1419.06140 194.82585 45.66134 18.06956 svd2$d # [1] 13.560545 2.736843 1.684743 1.335272 # u (or v) are also different
- Biplot
Number of components
Obtaining the number of components from cross validation of principal components regression
AIC/BIC in estimating the number of components
PCA and SVD
Using the SVD to perform PCA makes much better sense numerically than forming the covariance matrix to begin with, since the formation of [math]\displaystyle{ X X^T }[/math] can cause loss of precision.
- http://math.stackexchange.com/questions/3869/what-is-the-intuitive-relationship-between-svd-and-pca
- Relationship between SVD and PCA. How to use SVD to perform PCA?
R example
covMat <- matrix(c(4, 0, 0, 1), nr=2)
p <- 2
n <- 1000
set.seed(1)
x <- mvtnorm::rmvnorm(n, rep(0, p), covMat)
svdx <- svd(x)
result= prcomp(x, scale = TRUE)
summary(result)
# Importance of components:
# PC1 PC2
# Standard deviation 1.0004 0.9996
# Proportion of Variance 0.5004 0.4996
# Cumulative Proportion 0.5004 1.0000
# It seems scale = FALSE result can reflect the original data
result2 <- prcomp(x, scale = FALSE) # DEFAULT
summary(result2)
# Importance of components:
# PC1 PC2
# Standard deviation 2.1332 1.0065 ==> Close to the original data
# ==> How to compute
# sqrt(eigen(var(x))$values ) = 2.133203 1.006460
# Proportion of Variance 0.8179 0.1821 ==> How to verify
# 2.1332^2/(2.1332^2+1.0065^2) = 0.8179
# Cumulative Proportion 0.8179 1.0000
result2$sdev
# 2.133203 1.006460 # sqrt( SS(distance)/(n-1) )
result2$sdev^2 / sum(result2$sdev^2)
# 0.8179279 0.1820721
result2$rotation
# PC1 PC2
# [1,] 0.9999998857 -0.0004780211
# [2,] -0.0004780211 -0.9999998857
result2$sdev^2 * (n-1)
# [1] 4546.004 1011.948 # SS(distance)
# eigenvalue for PC1 = singular value^2
svd(scale(x, center=T, scale=F))$d
# [1] 67.42406 31.81113
svd(scale(x, center=T, scale=F))$d ^ 2 # SS(distance)
# [1] 4546.004 1011.948
svd(scale(x, center=T, scale=F))$d / sqrt(nrow(x) -1)
# [1] 2.133203 1.006460 ==> This is the same as prcomp()$sdev
svd(scale(x, center=F, scale=F))$d / sqrt(nrow(x) -1)
# [1] 2.135166 1.006617 ==> So it does not matter to center or scale
svd(var(x))$d
# [1] 4.550554 1.012961
svd(scale(x, center=T, scale=F))$v # same as result2$rotation
# [,1] [,2]
# [1,] 0.9999998857 -0.0004780211
# [2,] -0.0004780211 -0.9999998857
sqrt(eigen(var(x))$values )
# [1] 2.133203 1.006460
eigen(t(scale(x,T,F)) %*% scale(x,T,F))$values # SS(distance)
# [1] 4546.004 1011.948
sqrt(eigen(t(scale(x,T,F)) %*% scale(x,T,F))$values ) # Same as svd(scale(x.T,F))$d
# [1] 67.42406 31.81113.
# So SS(distance) = eigen(t(scale(x,T,F)) %*% scale(x,T,F))$values
# = svd(scale(x.T,F))$d ^ 2
Related to Factor Analysis
- http://www.aaronschlegel.com/factor-analysis-introduction-principal-component-method-r/.
- http://support.minitab.com/en-us/minitab/17/topic-library/modeling-statistics/multivariate/principal-components-and-factor-analysis/differences-between-pca-and-factor-analysis/
In short,
- In Principal Components Analysis, the components are calculated as linear combinations of the original variables. In Factor Analysis, the original variables are defined as linear combinations of the factors.
- In Principal Components Analysis, the goal is to explain as much of the total variance in the variables as possible. The goal in Factor Analysis is to explain the covariances or correlations between the variables.
- Use Principal Components Analysis to reduce the data into a smaller number of components. Use Factor Analysis to understand what constructs underlie the data.
prcomp vs princomp
prcomp vs princomp from sthda. prcomp() is preferred compared to princomp().
Missing data
Relation to Multidimensional scaling/MDS
With no missing data, classical MDS (Euclidean distance metric) is the same as PCA.
Comparisons are here.
Differences are asked/answered on stackexchange.com. The post also answered the question when these two are the same.
isoMDS (Non-metric)
cmdscale (Metric)
Matrix factorization methods
http://joelcadwell.blogspot.com/2015/08/matrix-factorization-comes-in-many.html Review of principal component analysis (PCA), K-means clustering, nonnegative matrix factorization (NMF) and archetypal analysis (AA).
Outlier samples
Detecting outlier samples in PCA
Reconstructing images
Reconstructing images using PCA
Sparse PCA
- https://en.wikipedia.org/wiki/Sparse_PCA
- 8.6.1 Sparse Principal Components from Harrell's "Regression Modeling Strategies" book
- pcaPP package
Misc
PCA for Categorical Variables
- FAMD - Factor Analysis of Mixed Data in R: Essentials
- PCA for Categorical Variables in R. Factorial Analysis of Mixed Data (FAMD) Is a PCA for Categorical Variables Alternate.
psych package
Principal component analysis (PCA) in R
glmpca: Generalized PCA for dimension reduction of sparse counts
Feature selection and dimension reduction for single-cell RNA-Seq based on a multinomial model